About us
Building on a tradition of excellence that dates back more than one hundred years, the CommonSpirit Health Research Institute (CSHRI) is focused on connecting hospitals, participants and physicians with the latest in medical advances, technology, treatment and procedures.
Led by a strong diverse team of experienced, mission driven health care leaders, the CSHRI provides start-to-finish clinical research services, resources and clinical trials oversight across the enterprise. The research institute sponsors over 92 care sites and more than 650 clinical trials in the United States, resulting in approximately 4,000 annual participants and increased access to cancer research through the NCI Community Oncology Research Program (NCORP).

Mission-driven leadership
Vani Nilakantan, PhD
Dr. Vani Nilakantan is the System Vice President of the CommonSpirit Research Institute and has responsibility for research programs across the enterprise.


Research starts with you

News & participant stories
Clinical research service lines

Clinical research reach
CommonSpirit Health Research Institute
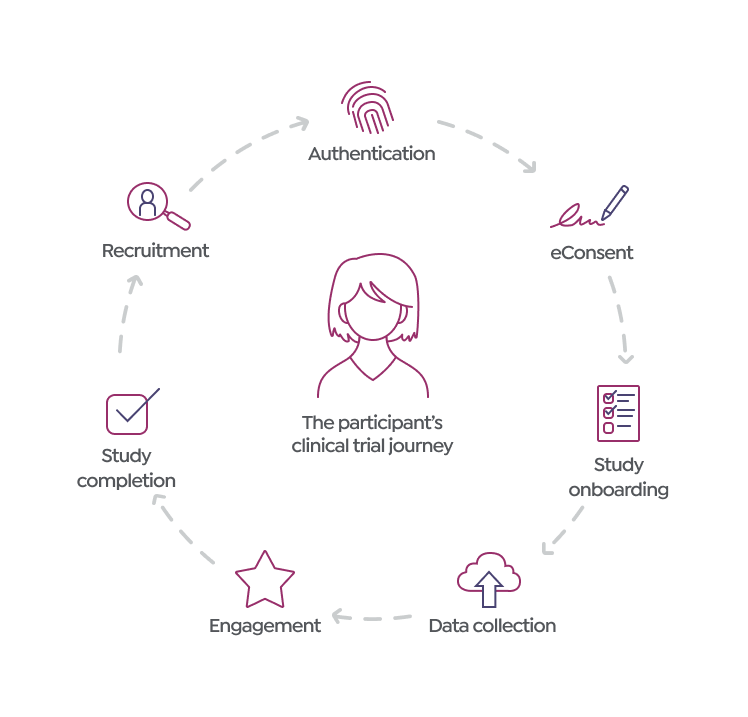
The participant’s clinical trial journey
- Recruitment: Potential research participants are identified and invited based on eligibility criteria to ensure a match with study requirements.
- Authentication: Verifying identity and eligibility for participation.
- Informed consent: Research participants sign consent forms, acknowledging their understanding and agreement to the terms of the study.
- Study onboarding: Getting familiarized with the study protocol, procedures, and expectations.
- Data collection: Providing necessary information and undergoing assessments as per the study requirements.
- Engagement: Staying involved and responsive throughout the study duration.
- Study completion: Finishing all study activities and assessments, marking the end of participation in the trial.
Annual reports
Ways to give
The Research Excellence Equity Fund (REEF) supports the intersectionality of research and health equity through initiatives like the Clinical Research Innovation Grant Program, which funds innovative, participant-centered research with a health equity focus, and strategies to enhance diversity in clinical trial participation. It also promotes diversity in the research workforce by supporting diverse medical students, residents, and fellows, and provides start-up funds for research to increase the recruitment of physicians of color.









